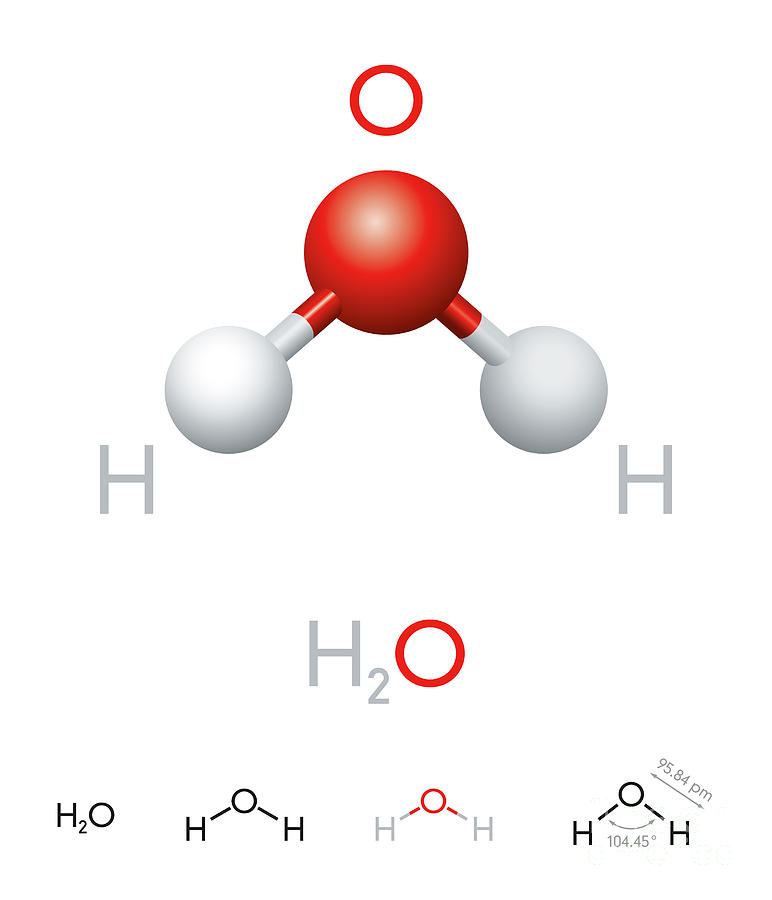
H2o Molecular Structure My XXX Hot Girl
Hi Guys!Today in this video we will help you determine the molecular geometry of H2O, also known as water. To know its molecular geometry we first look at it.

H2O Molecular Geometry and Bond Angles Molecular geometry, Molecular, Science projects
A quick explanation of the molecular geometry of H2O (Water) including a description of the H2O bond angles..more.more Lewis Structures, Introduction, Formal Charge, Molecular.
New What Is The Molecular Geometry Of H2O PNG GM
A quick explanation of the molecular geometry of H2O including a description of the H2O bond angles. Note. the precise bond angle is 104.5.Looking at the H2O.

H2O Molecular Geometry / Shape and Bond Angles YouTube
Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.

H2O Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Explanation: First, draw the Lewis structure of water: Since the O atom has two bonds and two lone pairs, its steric number is 4. The following table shows VSEPR structures based on a compound's steric number and the number of lone pairs: Since the steric number is 4 and there are two lone pairs, water has bent geometry. Answer link.

Lewis Dot Diagram For H2o Free Diagram For Student
Construct SALCs and the molecular orbital diagram for H 2 2 O. Preliminary Steps. Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principal axis. Step 2. Identify and count the pendant atoms' valence orbitals. Generate SALCs. Step 3. Generate the Γ Γ 's.
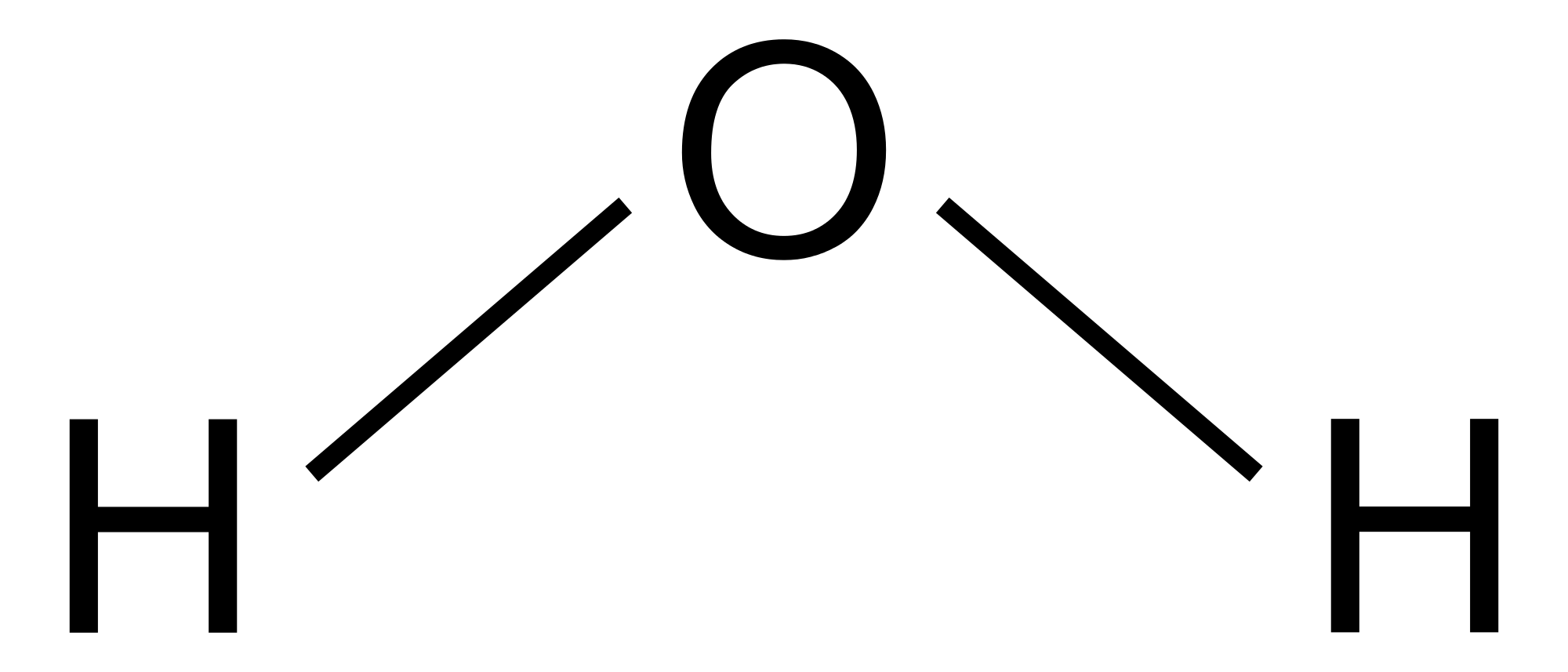
Estrutura De Lewis H2o ENSINO
Figure 5.9.9: (a) H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.9.3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

H2O Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
What is the molecular shape of H2O? How many lone pairs are in H2O? Why does h2o have lone pairs? Why H2O is not linear? The polarity of the molecules Lewis Structure and Molecular Geometry What are the valence electrons of hydrogen and oxygen

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
Chemistry model of molecule water H2O scientific elements. Integrated particles hydrogen and
Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the VSEPR theory. A table of geometries using the VSEPR theory can facilitate drawing and understanding molecules. The table of molecular geometries can be found in the first figure. The second figure serves as a visual aid for the table.

Water Molecule H2O Fiber Arts Art & Collectibles
© 2023 Google LLC How to find the molecular geometry for the H2O molecule (water).Join this channel to get full access to Dr. B's chemistry guides:https://www.youtube.com/chan.
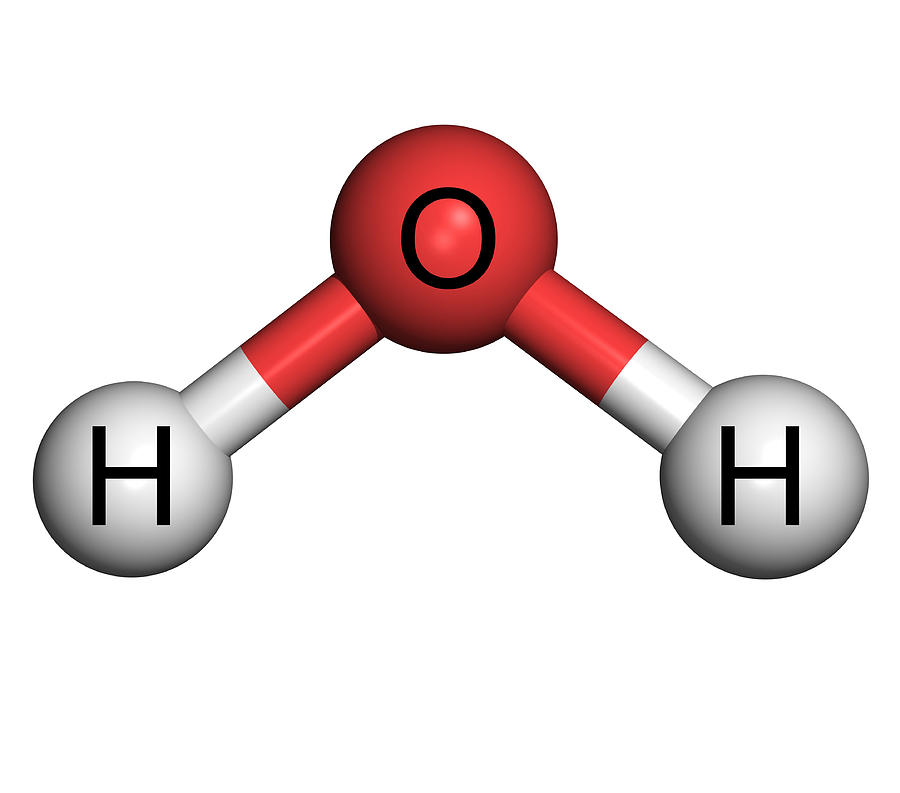
Молекула Воды Картинка Telegraph
Water (H2O) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. The Lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties. Steps to Draw the Lewis Structure of H2O Follow these steps to draw the Lewis structure of H2O:
Chemistry model of molecule water H2O scientific elements. Integrated particles hydrogen and
This charge polarization allows H 2 O to hydrogen-bond to other polarized or charged species, including other water molecules.. From this we can describe the molecular geometry. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple.

Molecular geometry of a water molecule. The molecular shape is an... Download Scientific Diagram
Understanding the Lewis structure and molecular geometry of H2O has several implications and applications in various fields of science. Knowing the Lewis structure and molecular geometry of H2O is crucial in chemistry. It predicts properties like polarity, boiling point, and solubility. H2O is an excellent solvent for polar molecules.
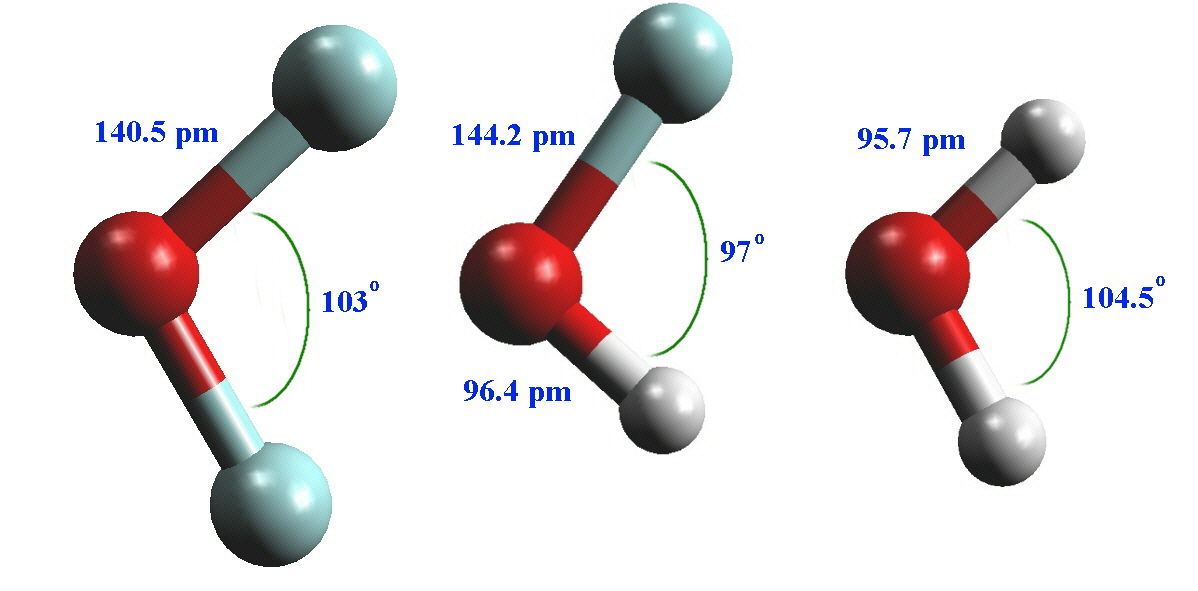
The Chemistry of the Halogens 4
Structure of Water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms.